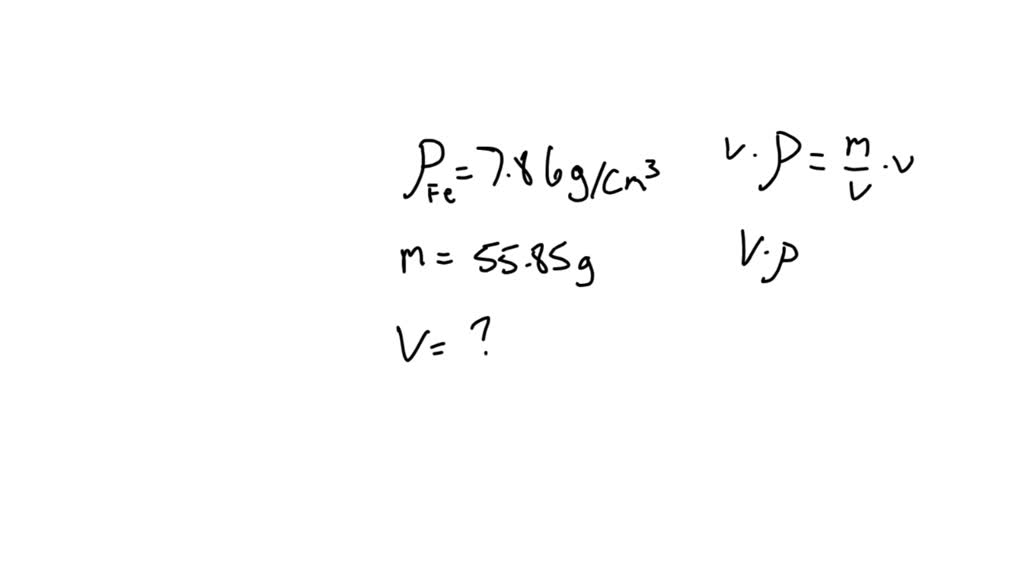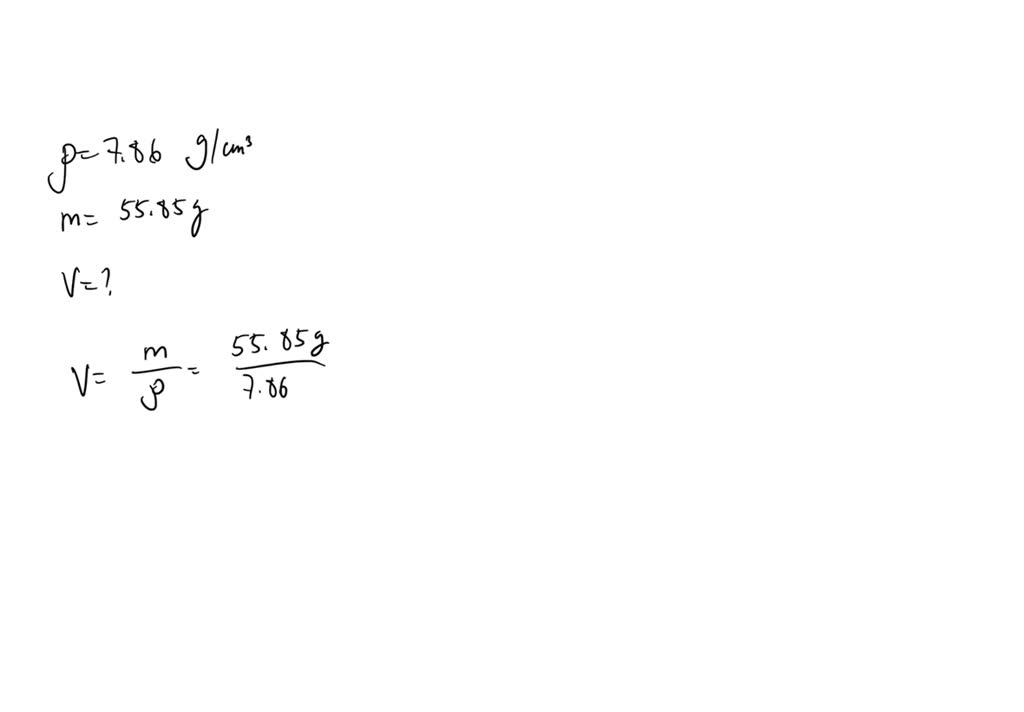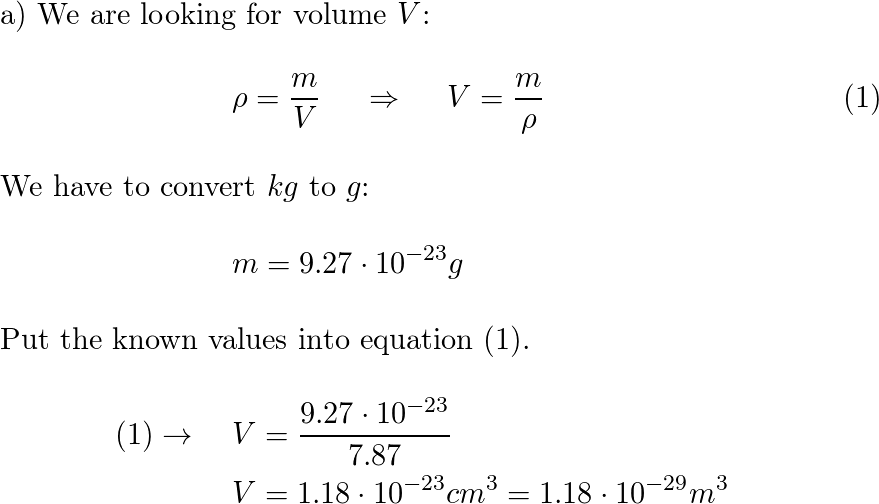Iron has a density of 7.86 g/cm3. Calculate the volume (in dL) of a piece of iron having a mass of 3.55 kg - Brainly.com
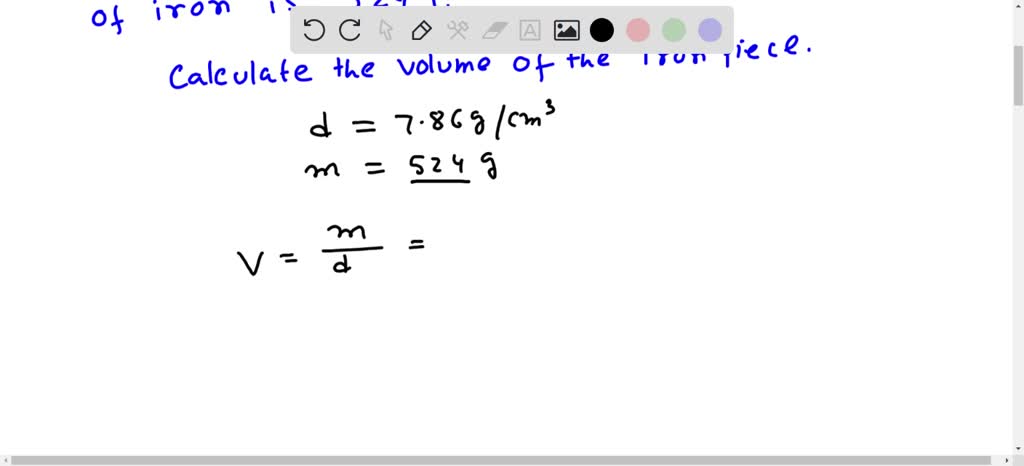
SOLVED: The density of iron is 7.86 g/cm3. What is the volume in milliliters (mL) of an irregularly shaped piece of iron that has a mass of 524 g?
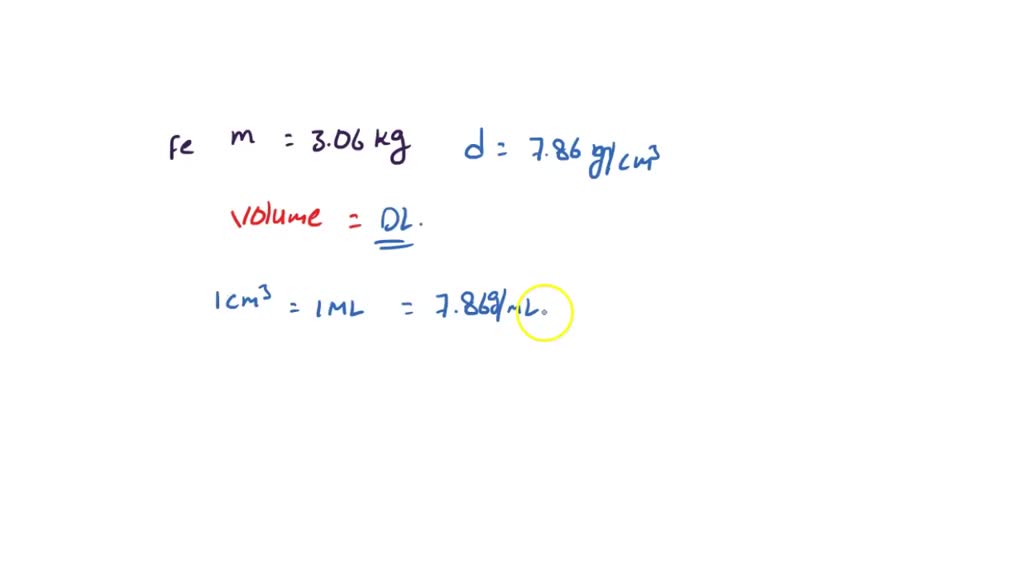
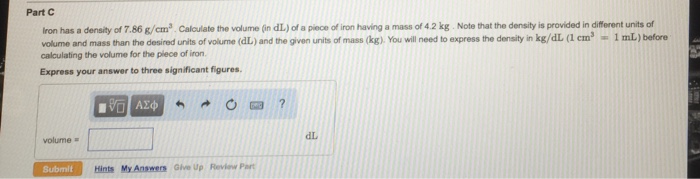
SOLVED: Iron has a density of 7.86 g/cm37.86 g/cm3. Calculate the volume (in dLdL) of a piece of iron having a mass of 3.06 kgkg . Note that the density is provided

Iron has an edge length 288 pm. Its density is 7.86 gm cm^-1 . Find the type of cubic lattice to which the crystal belongs. (Atomic mass of iron = 56 ).

Iron has a density of 7.86 g/cm3. Calculate the volume (in dL) of a piece of iron having a mass of 4.79 kg - Brainly.com

A unit cell of iron crystal has edge length 288 pm and density 7.86 g cm^-3 . Find the number of atoms per unit cell and type of the crystal lattice.Given: Molar
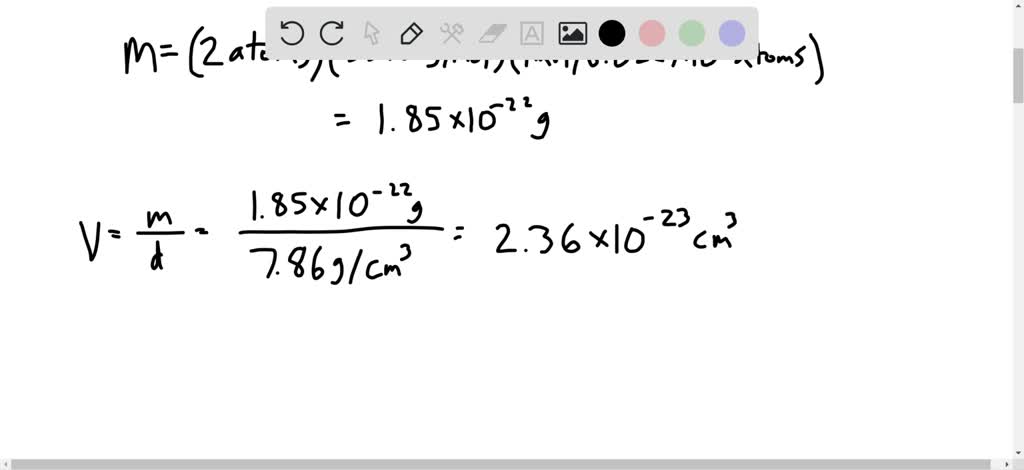
SOLVED:Iron has a density of 7.86 g / cm^3 and crystallizes in a bodycentered cubic lattice. Show that only 68 % of a body-centered lattice is actually occupied by atoms, and determine

Iron has a density of 7.86 g/cm3 (1 cm3=1 mL). Calculate the volume (in dL) of a piece of iron having a - Brainly.com
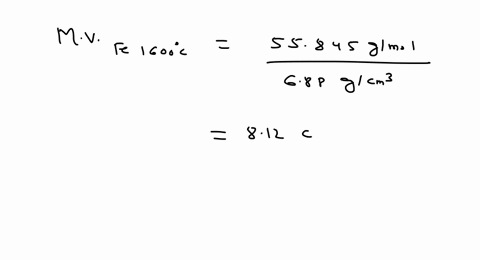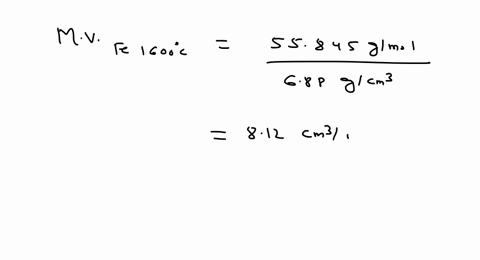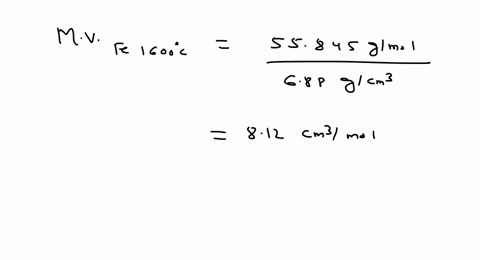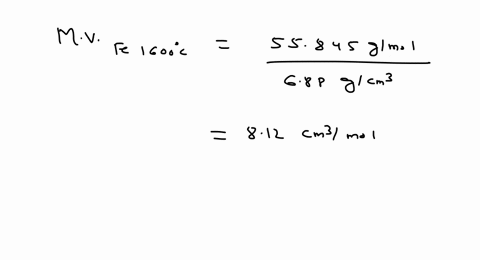
SOLVED: Iron has a density of 7.86 g/cm37.86 g/cm3. Calculate the volume (in dLdL) of a piece of iron having a mass of 3.06 kgkg . Note that the density is provided

A cannonball made of iron has a volume of 1115 cm^3. If iron has a density of 7.86g/cm^3, what is the mass - Brainly.com

Density Calculations Alysha Wenglarz - Chemistry I-1 2.6 Density Calculations Solve each of the following problems as directed. Show all of your work 1. | Course Hero

Iron has an edge length 288 pm. Its density is 7.86 gm cm^-1 . Find the type of cubic lattice to which the crystal belongs. (Atomic mass of iron = 56 ).